Early Drug Development. Strategies and Routes to First-in-Human Trials
Description
The focus of early drug development has been the submission of an Investigational New Drug application to regulatory agencies. Early Drug Development: Strategies and Routes to First-in-Human Trials guides drug development organizations in preparing and submitting an Investigational New Drug (IND) application. By explaining the nuts and bolts of preclinical development activities and their interplay in effectively identifying successful clinical candidates, the book helps pharmaceutical scientists determine what types of discovery and preclinical research studies are needed in order to support a submission to regulatory agencies.
Technical Details
| age: | 0 |
| author: | Mitchell Cayen N. |
| genres_list: | 5708,6434 |
| ISBN: | 9780470613177 |
| lang: | en |
| litres_isbn: | 978-5-04-477335-6 |
| publisher: | John Wiley & Sons Limited |
| Type: | book |
| Форматы: |
Price history chart & currency exchange rate
Customers also viewed

45.48 руб.
Acrylic Disc DIY Keychain Making Kit Goo Card Water Droplets DIY Goo Plate DIY Transparent Keychain Goo Card Kids Gift
aliexpress.com
2,014.23 руб.
Bit Changing Drill Chuck Replacement DIY Projects Cost-efficient Solution Metal And Plastic DHP484 DDF446 1 2''
aliexpress.com
739.91 руб.
GT1575-VTBA GT1575-VTBD Touch Panel Screen Glass Digitizer GT1575-VTBA GT1575-VTBD Protective Film Overlay
aliexpress.com
970.57 руб.
4150mAh Durable For Umi Umidigi A5 Pro A5Pro Reliable Power Supply Mobile Phone Battery
aliexpress.com
4,422.38 руб.
Коробка для рыболовных снастей, 4-уровневая коробка для аксессуаров для рыболовных снастей, портативный органайзер для рыболовных снастей, коробка для хранения снастей для морской рыбалки
aliexpress.com
79.59 руб.
Damper Air Vent Garden Outdoor 125mm Home Parts Replacement Test Chamber 1 X Vent Accessories BBQ Barbecue Tools
aliexpress.com
69.85 руб.
1Pc Baby Auricle Support External Auricle Corrector Protruding Baby Ear Corrector Baby External Auricle Corrector Ear Pads
aliexpress.com
3,758.82 руб.
2 комплекта, дружеский браслет со шнурком и бусинами в виде букв, цветная нить для вышивки, эластичный шнур, плетеный диск
aliexpress.ru
851.99 руб.
20204-AG011 втулка переднего нижнего рычага управления для Subaru FORESTER IMPREZA LEGACY OUTBACK XV Передняя подвесная втулка
aliexpress.com
1,870.48 руб.
Hip-hop jeans European and American high street Y2k men's style personality suspenders street black/blue straight loose jeans
aliexpress.com
1,091.58 руб.
2023 Oversized Mens T-shirt + Shorts Set Summer Breathable Casual T shirt Running Set Fashion Harajuku Printed Male Sport Suit
aliexpress.com
1,080.21 руб.
Aluminum alloy double PD port car charger fast car charger 30W mobile phone charger
aliexpress.com
333.81 руб.
New Magician Rabbit Metal Cutting Dies Scrapbook Embossed Make Paper Card Album Diy Craft Template Decoration Die 2022 Arrivals
aliexpress.ru
340.31 руб.
Electroplating Hot Stamping Glitter Love Case For Samsung Galaxy Z Fold 3 2 Cover For Samsung Fold Case For Samsung Z flip
aliexpress.ru
4,627.86 руб.
Материнская плата B75 8 карт для майнинга BTC + ЦП + вентилятор + термоподушка + отвертка + SATA + кабель переключателя 8X USB3.0(PCIE)LGA1155 DDR3 MSATA
aliexpress.ru
178.68 руб.
For OPPO ENCO Air 2 Pro Earphones Case Fashion 3D Cute Bear Flower Love Headphones Cover For OPPO Air 2 Pro TWS Headset Shell
aliexpress.com
211.17 руб.
Geometric Pattern Auto-absorbed Card Holder PU Leather Phone Shell for Samsung Galaxy M10/A10 - Coffee, Galaxy A10
tvc-mall.com
107,615.27 руб.
EZcad Marking Software IPG Expiry Date Code Laser Marking Machine Fiber Laser With Rotary System For Sale
aliexpress.com
825.00 руб.
Краска для сколов KIWIX 2в1 PEUGEOT 149F ROUGE GRENADE 20 мл, Красный, 2в1 PEUGEOT 149F ROUGE GRENADE 20 мл
goods.ru
848.00 руб.
Антивозрастной крем для рук Королевский лотос, масло Ши и жожоба (hand cream) Organic Tai | Органик Тай 120мл
ashaindia.ru
3,232.98 руб.
Mulheres Vestido de turno Minivestido Branco Manga Curta Cor imaculada Patchwork Primavera Verão Decote V Ajuste Largo 2022 S M L XL XXL
miniinthebox.com
2,548.88 руб.
água-viva astronauta luz negra uv reativo tapeçaria psicodélico trippy dormitório sala de estar arte decoração pano de suspensão
miniinthebox.com
2,563.95 руб.
Tableta Carcasa Funda Para Samsung Galaxy Tab S8 Plus S8 11'' A7 2022 2020 Portalápiz Soporte de Coche con Soporte Color sólido Cuero de PU
miniinthebox.com
1,341.81 руб.
3 stücke künstliche unterwasserpflanzen aquarium dekoration wasser gras anzeige dekorationen unkraut unterwasserpflanzen aquarium aquarium
miniinthebox.com
3,391.28 руб.
Damen Etuikleid Weißes Kleid skims dress Minikleid Weiß Ärmellos Blumen Taste Frühling Sommer V Ausschnitt Elegant Wochenende 2023 S M L XL XXL
miniinthebox.com
3,262.19 руб.
Bauerntischdecke Esstischdecke Frühlingstischdecke Runde Outdoor-Tuch Tischdecke Ovales Rechteck für Picknick, Hochzeit, Ostern, Küche
miniinthebox.com
11,740.79 руб.
shows glowing glasses with 10pcs red laser led stage luminescent club party decoration
dhgate.com
32,102.55 руб.
Шкаф купе Luxe Studio Стандарт-1 Фотопечать Природа-108 2300x2100x450 трехдверный
rozetka.com.ua
875.84 руб.
camisoles & tanks 62-84cm school student girls sport training bra spaghetti strap bandeau cami crop single layer ribbed solid color unde, Black;white
dhgate.com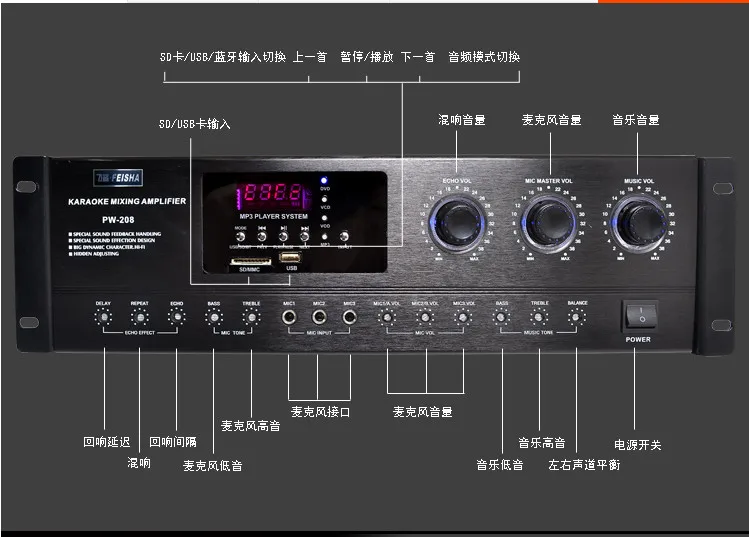
7,068.50 руб.
220V High-power professional KTV HIFI amplifier audio home karaoke Bluetooth amplifier stage PW-208 330w+330w
aliexpress.com
130.76 руб.
3-piece Nail Toenail Pedicure Tool Steel Nail Clippers Dead Pedicure Care Remove Foot Tool On Skin Feet To Set I3C9
aliexpress.com
6,401.25 руб.
harajuku hoodies men hip hop streetwear hoodie graffiti pullovers oversized 2020 autumn loose casual cotton hooded sweatshirts #at12, Black
dhgate.com
270.46 руб.
BaanSam New Colorful Battery Back Cover For Nokia Asha 502 Housing Case With Power Volume Buttons
aliexpress.com
77.16 руб.
Fashion Afro Girls Melanin Queen Case For LG X Power 2 Q7 Q6 V20 V30 K4 K8 K11 K10 2017 2018 G6 G5 G8 ThinQ Nexus 5X Soft Cases
aliexpress.com
866.61 руб.
100pcs gift bag Drawstring jewelry bunch mouth bag wenwan cosmetics gift bag 7x9cm
aliexpress.com











